Description
Description
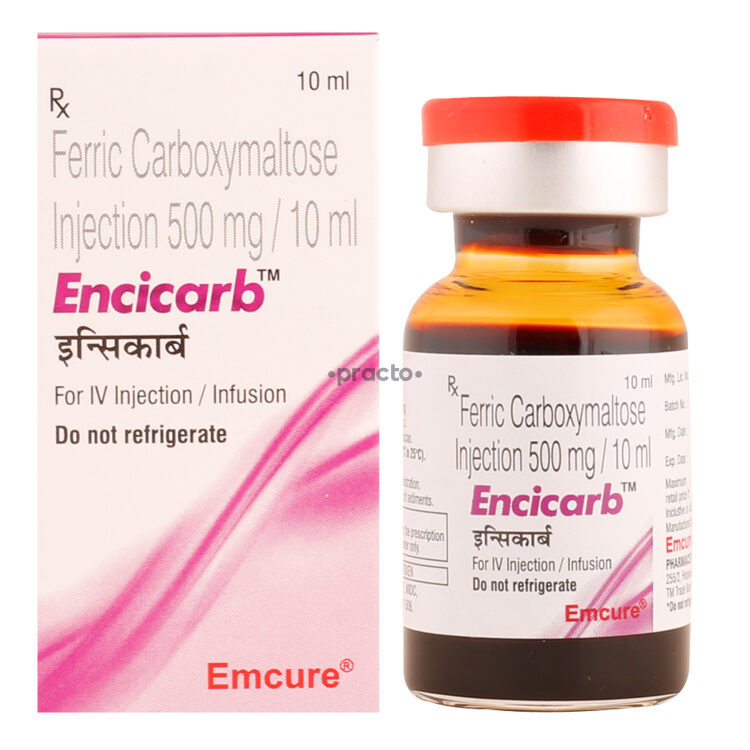
Encicarb 750mg injection contains the active substance Ferric carboxymaltose. It belongs to the group of medications called iron replacement products. It is used to treat iron-deficiency anemia (decreased RBC due to lower levels of iron in the body) in adults and pediatric patients who cannot tolerate oral iron or when oral iron supplements are ineffective. It is also indicated to treat iron deficiency anemia in adults with chronic kidney disease who are not on dialysis. This medicine is not recommended for pediatric patients less than one year of age.
Do not take this medicine if you are allergic to ferric carboxymaltose or any of the other ingredients of this medicine. Do not receive Encicarb 750mg injection if you have anemia that is not caused by iron deficiency, an iron overload (too much iron in the body), or difficulties in using iron. Inform your physician if you have rheumatoid arthritis, severe asthma, eczema, infection, liver disorders, or low phosphate levels in the blood. Incorrect administration of this injection may cause irritation and long-lasting discoloration at the administration site.
Inform your physician if you are pregnant or breastfeeding before initiating the treatment with Encicarb 750mg injection. The most common side effects are nausea, hypertension, flushing, injection site reactions, skin reactions, vomiting, and dizziness. Tell your physician if you have experienced serious allergic (hypersensitive) reactions to other injectable iron preparations.
Date of Approval
The FDA approved ferric carboxymaltose (Injectafer) for the treatment of iron deficiency anemia on July 25, 2013. The FDA approved Injectafer for other uses at later dates, including:
June 5, 2023: For the treatment of iron deficiency in adult patients with heart failure
December 16, 2021: For the treatment of iron deficiency anemia in pediatric patients
May 6, 2021: For the treatment of iron deficiency anemia in adult patients with a single dose option
Mechanism of Action of Ferric Carboxymaltose
Ferric carboxymaltose is a macromolecular ferric hydroxide carbohydrate complex, which allows for controlled delivery of iron within the cells of the reticuloendothelial system and subsequent delivery to the iron-binding proteins ferritin and transferrin.
Uses of Ferric Carboxymaltose
Ferric carboxymaltose injection is an iron replacement product that is used to treat iron deficiency anemia (not enough iron in the blood) in patients with non-dialysis dependent chronic kidney disease (CKD).
Ferric Carboxymaltose Dosage available
Encicarb 750mg injection is an intravenously administered prescription drug. This injection will be given to you in a hospital or clinic setting. Your doctor will decide the dose, duration, and frequency of the administration based on your condition and other factors. Your doctor may perform a blood test to determine how much medicine you need.
Which areas or states do you supply to?
Encicarb (Ferric Carboxymaltose) Injection is available in following cities in India – Mumbai, Delhi, Gurgaon, Noida, Bengaluru, Hyderabad, Ahmedabad, Chennai, Kolkata, Surat, Pune, Jaipur, Lucknow, Kanpur, Gurgaon, Punjab, Nagpur, Indore, Thane, Bhopal, Visakhapatnam, Pimpri-Chinchwad, Patna, Vadodara, Ghaziabad, Ludhiana, Agra, Nashik, Faridabad, Meerut, Rajkot, Kalyan-Dombivli, Vasai-Virar, Varanasi, Srinagar, Aurangabad, Dhanbad, Amritsar, Navi Mumbai, Prayagraj, Howrah, Ranchi, Jabalpur, Gwalior, Coimbatore, Vijayawada, Jodhpur, Madurai, Raipur, Kota, etc.
For large institutional or inter-state distribution, please contact our sales department for region-specific arrangements.