Description
Description
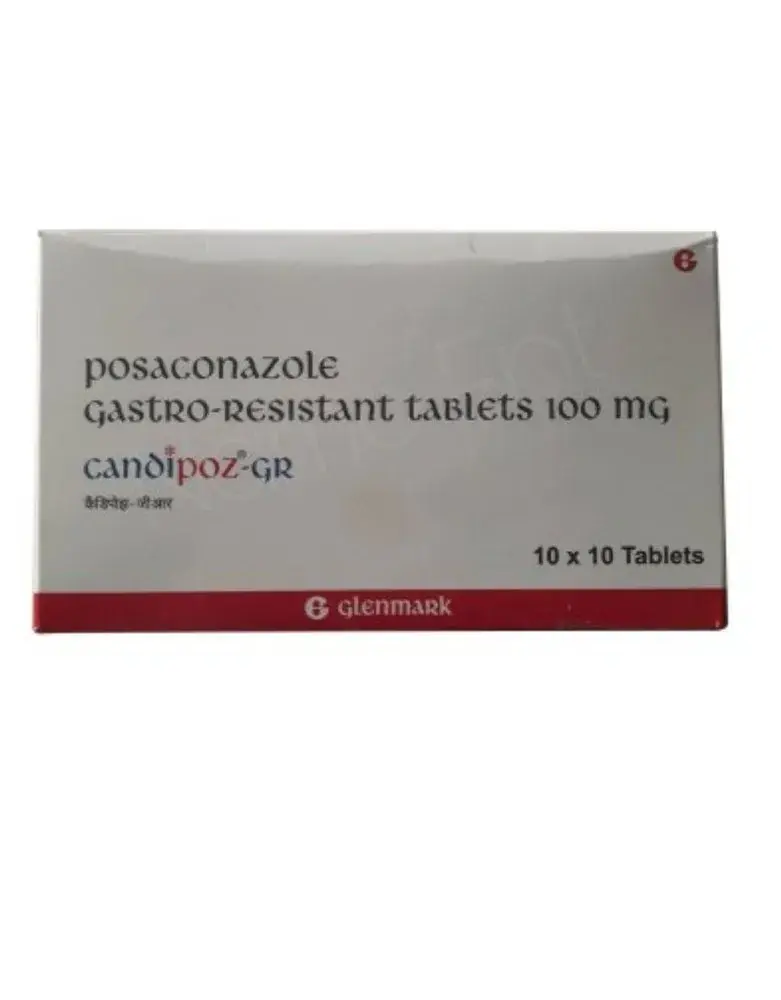
This page contains brief details about the drug posaconazole, it’s indication, dosage & administaration, mechanism of action, related brands with strength, warnings and common side effects.
Date of Approval
Posaconazole is a group of medicines called Azole antifungals. The U.S. FDA officially approved Posaconazole for its medical use in 2006.
Mechanism of Action of Posaconazole
Posaconazole inhibits ergosterol synthesis by binding and inhibiting the lanosterol-14alpha-demethylase enzyme, which is found in almost all fungi and is a key component of the fungal cell membrane. It stops the activity of the organisms responsible for fungus infections.
Uses of Posaconazole
Posaconazole is used to treat fungal infections, oropharyngeal candidiasis (OPC), and Prophylaxis of invasive aspergillus and candida infections in severely immunocompromised patients.
Posaconazole Dosage available
Posaconazole is available as an injection, delayed-release tablet, syrup or oral suspension. The recommended dose of Posaconazole tablet is 300 mg twice daily and must be taken with food. Avoid crushing, chewing, or breaking the tablets; swallow them whole. Posaconazole injection is usually given over 30 to 90 minutes in your vein. Take each oral suspension dose within 20 minutes after a full meal. A measured dosing spoon comes with your oral suspension and is marked for 2.5 ml and 5 ml doses. Follow your doctor’s instructions attentively and continue to take it at the dose and time suggested by your doctor. It may worsen your symptoms, so don’t stop taking it without first talking to your doctor.