Description
Description
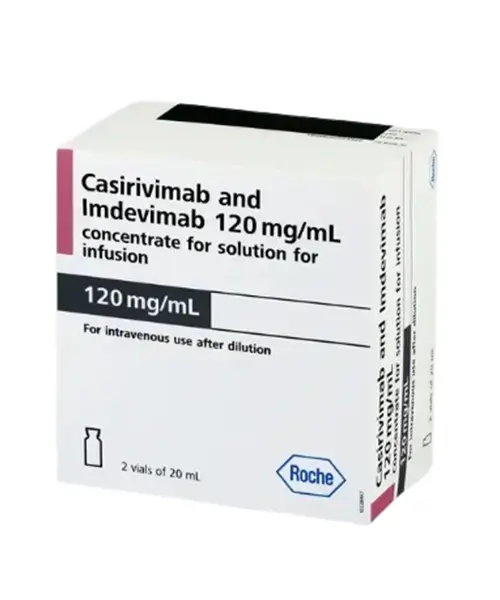
Ronapreve 120mg Injection contains the active constituent Casirivimab + Imdevimab, a type of protein called monoclonal antibodies. It is used to treat patients with confirmed acute COVID-19 infection. It is also indicated to prevent acute COVID-19 infection. Acute covid-19 infection is caused by a virus called coronavirus. People can get COVID-19 through contact with another person who has the virus.
Do not take Ronapreve 120mg Injection if you are allergic to Casirivimab + Imdevimab or any other ingredients. This medicine can cause allergic reactions following the infusion or injection; notify your physician immediately if you face any of the infusion reactions. Before you take this injection, inform your doctor if you have been vaccinated against COVID infection. Inform your doctor if you are pregnant or breastfeeding before initiating the treatment.
The side effects of this Ronapreve 120mg Injection monoclonal antibody therapy are injection site reactions, fever, flushing, shortness of breath, chest tightness, nausea, and vomiting. If you experience any of these side effects, immediately inform your doctor. This medicine is recommended to use in individuals aged 12 years and older and weighing at least 35.5kg.
Date of Approval
Casirivimab + Imdevimab is an antiviral drug that belongs to the class of medications called monoclonal antibodies. FDA approved it on November 21, 2020, for treating acute COVID-19 infection.
Mechanism of Action of Casirivimab Imdevimab
Casirivimab + Imdevimab are two recombinant human monoclonal antibodies that attach to a protein on the surface of the coronavirus called the ‘spike protein.’ This stops the virus from getting into your cells and causing an infection. They block the receptor binding domain (RBD) to the ACE2 receptor and prevent viral attachment to host cells. This can help your body to overcome the virus infection and may help you get better faster.
Uses of Casirivimab Imdevimab
Casirivimab + Imdevimab is available as an injection. It is used to treat patients with confirmed acute COVID-19 infection. It is also indicated to prevent acute COVID-19 infection. Acute covid-19 infection is caused by a virus called coronavirus. People can get COVID-19 through contact with another person who has the virus.
Casirivimab Imdevimab Dosage available
Casirivimab + Imdevimab is given as an infusion into your vein that lasts for 20 to 30 minutes, or it can be given as a subcutaneous (under the skin) injection. This injection will be given to you by a doctor or nurse who is experienced in the use of this type of treatment. They will monitor you closely while you are being given this medicine. Do not self-administer this medicine.
Which areas or states do you supply to?
Ronapreve (Casirivimab Imdevimab) Injection is available in following cities in India – Mumbai, Delhi, Gurgaon, Noida, Bengaluru, Hyderabad, Ahmedabad, Chennai, Kolkata, Surat, Pune, Jaipur, Lucknow, Kanpur, Gurgaon, Punjab, Nagpur, Indore, Thane, Bhopal, Visakhapatnam, Pimpri-Chinchwad, Patna, Vadodara, Ghaziabad, Ludhiana, Agra, Nashik, Faridabad, Meerut, Rajkot, Kalyan-Dombivli, Vasai-Virar, Varanasi, Srinagar, Aurangabad, Dhanbad, Amritsar, Navi Mumbai, Prayagraj, Howrah, Ranchi, Jabalpur, Gwalior, Coimbatore, Vijayawada, Jodhpur, Madurai, Raipur, Kota, etc.
For large institutional or inter-state distribution, please contact our sales department for region-specific arrangements.