Description
Description
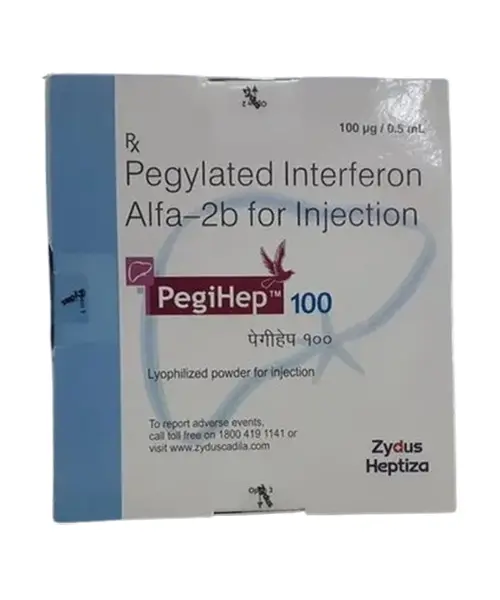
Pegihep 80mcg injection is an antineoplastic drug that belongs to the category of medicines known as interferons, containing the active ingredient Pegylated Interferon Alpha-2B. It is used to treat chronic hepatitis C infection in patients above 18 years of age with compensated liver disease. Hepatitis C infection is a viral infection that affects the liver. This infection can spread through contact with infected blood, by sharing needles or other drug injection equipment, or less commonly through sexual contact, sharing personal hygiene items, or from mother to baby during childbirth.
This medicine should be used with caution in patients with creatinine clearance below 50 mL/min, as this indicates moderate to severe impairment of renal function. This can lead to slower clearance of the medication, which may increase the risk of toxicity and adverse effects. The dosage of this drug should be reduced in these patients, and they should be closely monitored for signs of toxicity, such as fatigue, depression, or decreased white blood cell count. Therefore, the dose should be adjusted accordingly in patients with renal impairment. Tell your healthcare provider before beginning this treatment if you are pregnant or planning to have a baby. As this drug may cause harm to an unborn baby, it is advised for both men and women to follow proper contraceptive methods during and one week after the last dose of Pegihep 80mcg injection. Avoid breastfeeding your baby while you are on the treatment, as this drug can impact the growth and development of the baby by passing into the breast milk.
It is not recommended to take this injection if you are allergic to the drug or any of its ingredients. If you experience any signs of an allergic reaction, such as hives, difficulty breathing, or swelling of the face or throat, report to your doctor immediately. This drug is contraindicated in patients with decompensated liver disease. It is a serious condition where the liver is severely damaged and is no longer able to function properly, so it is highly advised to avoid taking this medicine.
Date of Approval
Mechanism of Action of Pegylated Interferon Alpha-2b
Approved 1986. Interferon alpha (IFN-α), a cytokine that is responsible for regulating and activating the immune response, was the first cancer immunotherapy approved by the FDA in 1986.
Uses of Pegylated Interferon Alpha-2b
Chronic Hepatitis C (CHC).
Pegylated Interferon Alpha-2b Dosage available
Pegihep 80mcg injection injection is administered as a subcutaneous injection (under your skin). If this drug is recommended for you, it will be given by a doctor. Do not self-administer. the recommended dose is 1.5 micrograms/kg/week. Your doctor will choose the dosage and administration frequency based on the condition because it differs from person to person.
Which areas or states do you supply to?
Pegihep (Pegylated Interferon Alpha-2b) Injection is available in following cities in India – Mumbai, Delhi, Gurgaon, Noida, Bengaluru, Hyderabad, Ahmedabad, Chennai, Kolkata, Surat, Pune, Jaipur, Lucknow, Kanpur, Gurgaon, Punjab, Nagpur, Indore, Thane, Bhopal, Visakhapatnam, Pimpri-Chinchwad, Patna, Vadodara, Ghaziabad, Ludhiana, Agra, Nashik, Faridabad, Meerut, Rajkot, Kalyan-Dombivli, Vasai-Virar, Varanasi, Srinagar, Aurangabad, Dhanbad, Amritsar, Navi Mumbai, Prayagraj, Howrah, Ranchi, Jabalpur, Gwalior, Coimbatore, Vijayawada, Jodhpur, Madurai, Raipur, Kota, etc.
For large institutional or inter-state distribution, please contact our sales department for region-specific arrangements.